Seawater Desalination
WET offers desalination systems for commercial, industrial and municipal use.
Reverse osmosis (RO) is a water purification process that uses a partially permeable membrane to remove ions, unwanted molecules and larger particles from drinking water. In reverse osmosis, an applied pressure is used to overcome osmotic pressure, a colligative property, that is driven by chemical potential differences of the solvent, a thermodynamic parameter. Reverse osmosis can remove many types of dissolved and suspended chemical species as well as biological ones (principally bacteria) from water, and is used in both industrial processes and the production of potable water. The result is that the solute is retained on the pressurized side of the membrane and the pure solvent is allowed to pass to the other side. To be “selective”, this membrane should not allow large molecules or ions through the pores (holes), but should allow smaller components of the solution (such as solvent molecules, i.e., water, H2O) to pass freely.
In the normal osmosis process, the solvent naturally moves from an area of low solute concentration (high water potential), through a membrane, to an area of high solute concentration (low water potential). The driving force for the movement of the solvent is the reduction in the free energy of the system when the difference in solvent concentration on either side of a membrane is reduced, generating osmotic pressure due to the solvent moving into the more concentrated solution. Applying an external pressure to reverse the natural flow of pure solvent, thus, is reverse osmosis. The process is similar to other membrane technology applications.
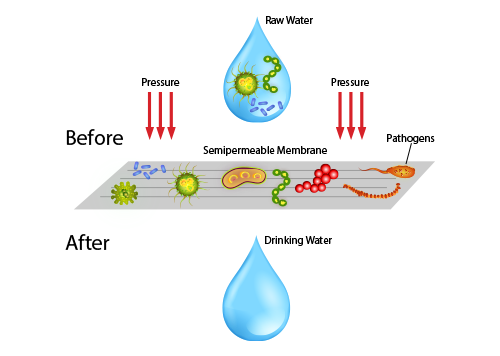
Reverse osmosis (RO) differs from filtration in that the mechanism of fluid flow is by osmosis across a membrane. The predominant removal mechanism in membrane filtration is straining, or size exclusion, where the pores are 0.01 micrometers or larger, so the process can theoretically achieve perfect efficiency regardless of parameters such as the solution’s pressure and concentration. Reverse osmosis instead involves solvent diffusion across a membrane that is either nonporous or uses nanofiltration with pores 0.001 micrometers in size. The predominant removal mechanism is from differences in solubility or diffusivity, and the process is dependent on pressure, solute concentration, and other conditions. Reverse osmosis is most commonly known for its use in drinking water purification from seawater, removing the salt and other effluent materials from the water molecules.
© 2018 MCI